Challenges with Existing Nanocarriers
Lipid nanoparticles (LNPs)
Lipid nanoparticles (LNPs) have been widely recognized for their role in drug delivery, particularly for mRNA vaccines, but they also present notable limitations. One major issue is their reliance on injectable administration, which can be inconvenient and uncomfortable for patients. Additionally, LNPs often accumulate in the liver, limiting their ability to circulate and deliver drugs to other parts of the body.
Read More
- Toxicity and Immune Responses: LNPs often concentrate in the liver and can be toxic to cells and may trigger unwanted immune reactions, leading to side effects.
- PEG-Related Immunogenicity: Polyethylene glycol (PEG), commonly used in LNP formulations, can cause immune responses on its own, independent of the lipid carrier.
- Manufacturing and Storage: LNPs require expensive cold-chain storage and specialized manufacturing processes, increasing costs and logistical complexity.
- Patent Issues: Freedom to operate can be a concern due to ongoing patent litigation, which may hinder innovation and accessibility.
- Limited Mucosal Delivery: LNPs struggle to cross mucosal barriers, restricting their suitability for non-invasive routes like oral or nasal delivery.
While LNPs have demonstrated success, addressing these challenges remains a priority in the field.
Virus-Like Particles (VLPs)
Virus-like particles (VLPs) are another nanocarrier system with potential, but they face several hurdles in drug delivery. Like LNPs, VLPs often require injectable administration, which can be a barrier to patient compliance. They also face issues with immune recognition, as the body may perceive them as foreign invaders, triggering immune responses that can lead to side effects or reduce their effectiveness.
Read More
- Manufacturing Complexity: Producing VLPs is expensive and requires specialized facilities, making large-scale production difficult.
- Cold-Chain Dependency: VLPs often need cold-chain storage, adding to costs and limiting their use in resource-limited settings.
- Liver Accumulation: Like LNPs, VLPs tend to accumulate in the liver, which can restrict their ability to target other tissues.
- Limited Cargo Capacity: VLPs have constraints on the size and type of cargo they can carry, which may limit their applicability for certain therapies.
While VLPs offer unique advantages, such as their ability to mimic viral structures for targeted delivery, these challenges underscore the need for continued innovation to overcome their limitations.
PNP Technology
Phoreus Biotech specializes in pioneering peptide-based nanocarrier technologies designed to enhance the delivery and efficacy of various therapeutics. Our proprietary platforms, including Branched Amphipathic Peptide Capsules (BAPC®️), Corralling Amphipathic Peptide Colloids (CAPC™️), and Amphipathic Peptide Capsules (APC), offer versatile and efficient solutions for targeted drug delivery. These self-assembling nanocarriers are engineered to overcome traditional absorption challenges, providing improved stability, biocompatibility, and the potential to revolutionize treatments across multiple medical fields.
Enhanced Safety of PNPs vs LNPs:
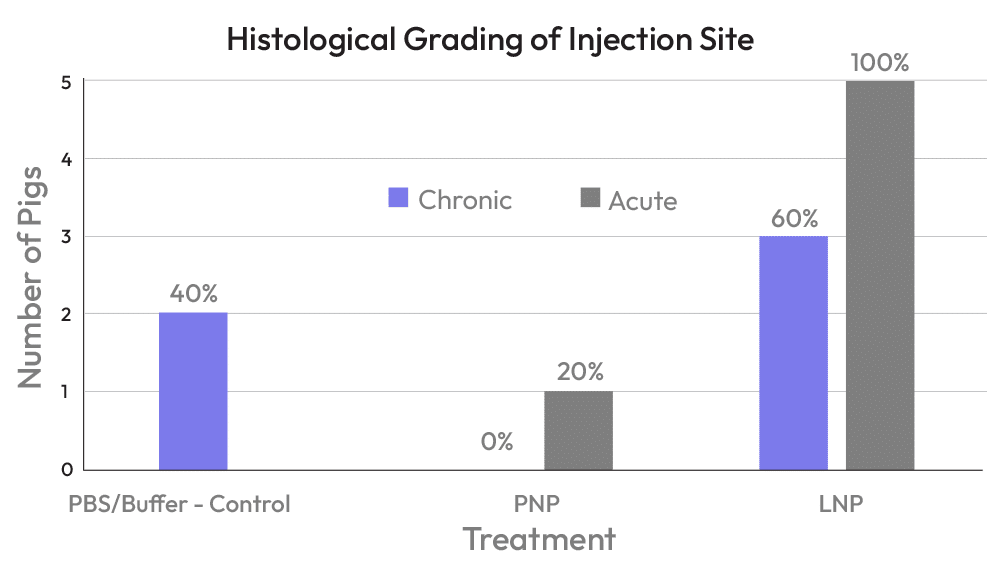
- Pilot pig toxicology study to assess the innate immune response of PNPs versus LNPs
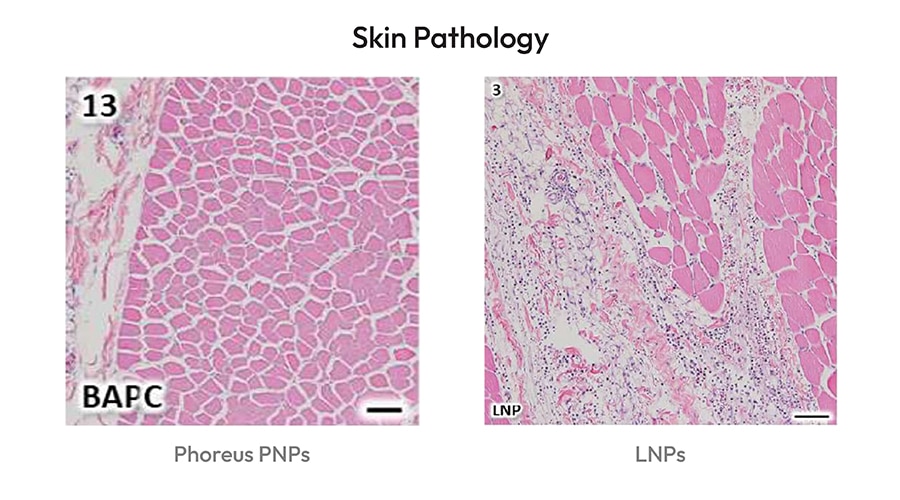
- The study involved 2 injections; evaluations included analysis of skin pathology and serum cytokines
- We demonstrate the PNPs elicit no response, while LNPs elicit an inflammatory response both locally and systemically
- 100% of LNP treated pigs presented with an acute or chronic reaction
- 0% of PNP treated pigs presented with a chronic reaction. Only 20% (1/5) of PNP treated pigs responded with an acute reaction
Applications
Phoreus Biotech’s peptide-based nanocarriers (PNPs) offer a next-generation approach to pulmonary drug delivery, enhancing stability, cellular uptake, and therapeutic precision to overcome key barriers in treating respiratory conditions like cystic fibrosis and COPD.
Phoreus Biotech’s peptide-based nanocarriers (PNPs) offer a breakthrough in fungicide delivery, enhancing bioavailability, reducing side effects, and eliminating the need for dose escalations – making therapies for this difficult-to-treat class more effective, accessible, and patient-friendly.
Phoreus Biotech’s peptide-based nanocarriers (PNPs) offer a next-generation solution for nucleic acid delivery, enhancing stability, targeting precision, and manufacturability to improve the accessibility and effectiveness of mRNA and DNA-based therapies.

Cell Transfection Kits
BAPtofect®️-25 kits are 25 nm conformationally locked BAPC for use in cell transfection research. Choose between general transfection and insect transfection kits that help overcome limitations associated with polymer-based and lipid products.
Shop Cell Transfection Kits
Find the right kit for your application.