What Are Nanoparticles?
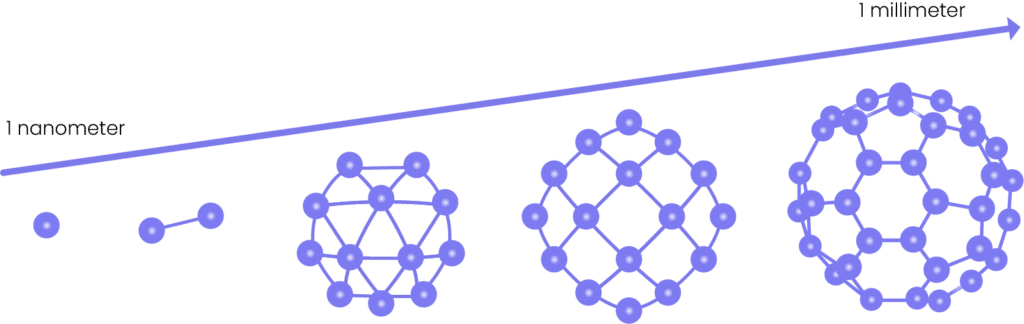
Nanoparticles are tiny particles with dimensions in the nanometer range measuring one billionth of a meter. Their size is so incredibly small that they remain invisible to the naked eye, and even conventional microscopes face challenges in their detection. The nanoparticle definition may vary depending on the context. Still, they are generally understood to be so small that they exhibit different physical and chemical properties to their bulk material counterparts.
Manufacturers make these particles using various materials, such as metals, semiconductors, ceramics, and polymers. Controlling their size, shape, and surface characteristics allows for tailoring their properties to their application. Due to their small size and distinctive properties, nanoparticles hold vast potential for applications across diverse fields such as medicine, electronics, energy, and environmental science.
In medicine, nanoparticle technology emerges as one of the most promising areas of research. Nanoparticles can be designed to target cancer cells, deliver drugs more efficiently, and improve the effectiveness of imaging techniques. Researchers can use them to develop biosensors and diagnostic devices with high sensitivity and specificity.
Nanoparticle Size
Nanoparticles typically range from 1 to 100 nanometers in size, which is about 1000 times smaller than the width of a human hair. Size is a crucial factor that affects nanoparticle properties and behavior. When the size of particles reduces to the nanoscale, their physical, chemical, and optical properties can change significantly. In general, as the size of nanoparticles decreases, their surface area to volume ratio increases, leading to a greater number of surface atoms and higher reactivity. This higher reactivity of smaller nanoparticles results from higher surface energy, making them ideal for catalysis and other chemical applications. Optical properties of nanoparticles, such as absorption, fluorescence, and scattering, are also size-dependent in this way.
Additionally, the size of nanoparticles can also affect their biological properties, such as their ability to cross biological barriers or interact with cells. Smaller nanoparticles can penetrate deeper into tissues, which may be desirable for drug delivery applications. However, it is crucial to carefully consider the potential risks associated with nanoparticles, as their small size may also increase their toxicity and environmental impacts.
How Are Nanoparticles Made?
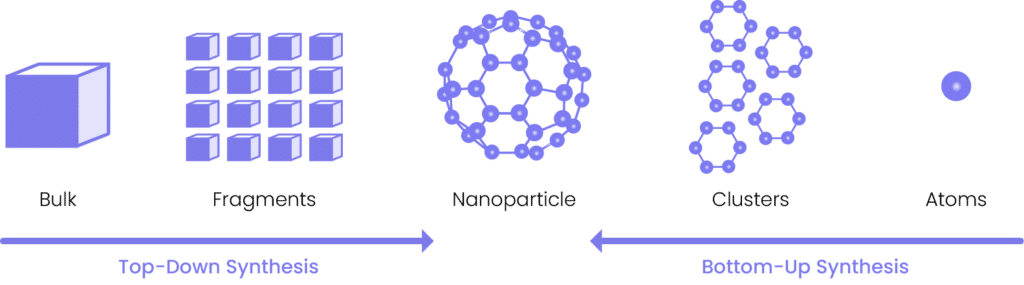
Nanoparticles can be made from an assortment of materials, including metals, metal oxides, ceramics, semiconductors, polymers, and biological lipids. They are engineered using one of two methods: top-down or bottom-up synthesis. In the top-down approach, bulk materials are broken down into smaller particles through milling, lithography, and etching. In contrast, the bottom-up approach involves building up nanoparticles from smaller building blocks like atoms or molecules using techniques such as (1) chemical synthesis, (2) self-assembly, or (3) biomineralization.