Researchers use transfection to study gene function, protein expression, and cellular processes. It is an important laboratory technique with wide-ranging applications in various fields, including biomedical research, drug discovery, drug delivery, and gene therapy development.
What Is Transfection?
Cell transfection refers to a collection of scientific and clinical research methods that are used to introduce foreign genetic material into cells. Scientists use transfection to transfer artificially engineered gene constructs and plasmids into eukaryotic cells to alter their protein expression, significantly changing their function.
Once the cell has been altered, researchers can investigate gene function, study disease mechanisms, develop potential treatments, and produce valuable proteins for medical and industrial purposes. Given the wide variety of applications, several different transfection methods have been developed. However, each has strengths and weaknesses optimized for the nucleic acid quantity and type of the cell being transfected for a particular use case.
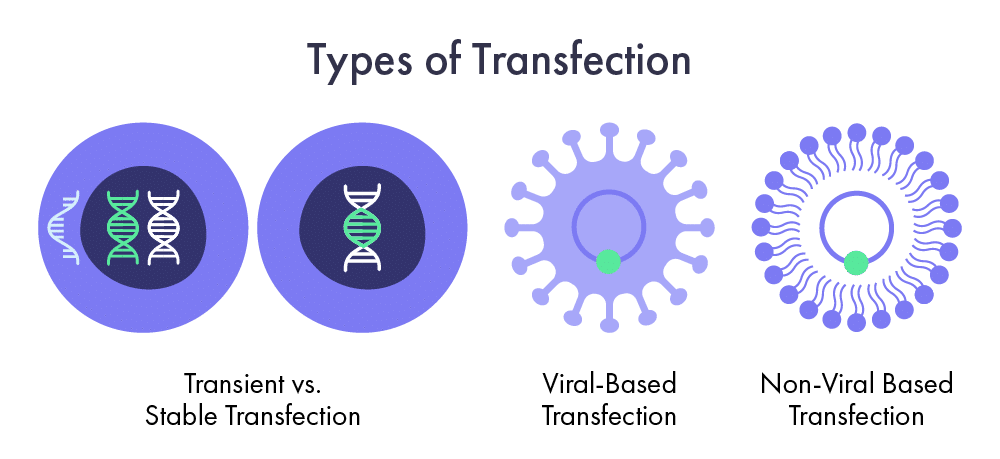
Types of Transfection Protocols
Transient vs. Stable Transfection
To explore the different facets of cell transfection, researchers often choose between two primary protocols: transient and stable transfection. The choice between these protocols depends on the specific research goals and experimental requirements.
Transient transfection is often used for short-term studies and rapid gene function or protein expression analysis. In contrast, stable transfection is employed when long-term or heritable expression of the introduced genetic material is desired, such as with gene therapy.
Transient Cell Transfection
Transient cell transfection refers to when foreign genetic material remains in cells for only a short time. The introduced RNA or DNA constructs do not integrate into the host cell’s genome. Instead, it remains separate from the cellular DNA. Transient transfection typically leads to temporary expression of the introduced genetic material, which may last a few days to a few weeks, depending on the cell type and experimental conditions. During this transient expression, the introduced genetic material is used to study short-term effects, observe protein expression, or perform functional assays. However, the introduced genetic material is diluted or degraded within the cells over time, and its expression diminishes.
Stable Cell Transfection
On the other hand, stable cell transfection involves the long-term integration of the introduced genetic material into the host cell’s genome. In this approach, the foreign DNA is usually designed to integrate into a specific genomic location within the host cell. The integration can occur randomly or be targeted to a particular site using techniques like homologous recombination. Once integrated, the genetic material becomes a permanent part of the host cell’s genome and is replicated along with the cellular DNA during cell division. Stable transfection allows for the sustained and heritable expression of the introduced genetic material in subsequent generations of cells. This is particularly useful when studying long-term effects, establishing cell lines with stable gene expression, or generating genetically modified organisms.