Transdermal drug delivery products have been around for many years but are limited to lipophilic drugs with low molecular weight that are effective at low doses. Research is currently underway to develop transdermal drugs that can more effectively deliver large doses of hydrophilic drugs for disease treatment and vaccination.
Transdermal drug delivery offers many advantages over more traditional oral and intravenous delivery methods. Not only are they less invasive than injections, but they can easily be administered outside of medical institutions. They also offer the advantage of not losing their effectiveness due to the first-pass effect of the liver. Current transdermal drugs suffer from requiring high potency to overcome the skin barrier that can often cause irritation and other damage to the skin.
Nanocarriers offer a promising way to increase the penetration capabilities of drugs/vaccines and better control their release. These new nanocarrier-based delivery methods have the potential to completely transform the effectiveness of transdermal delivery for a wide variety of therapeutics.
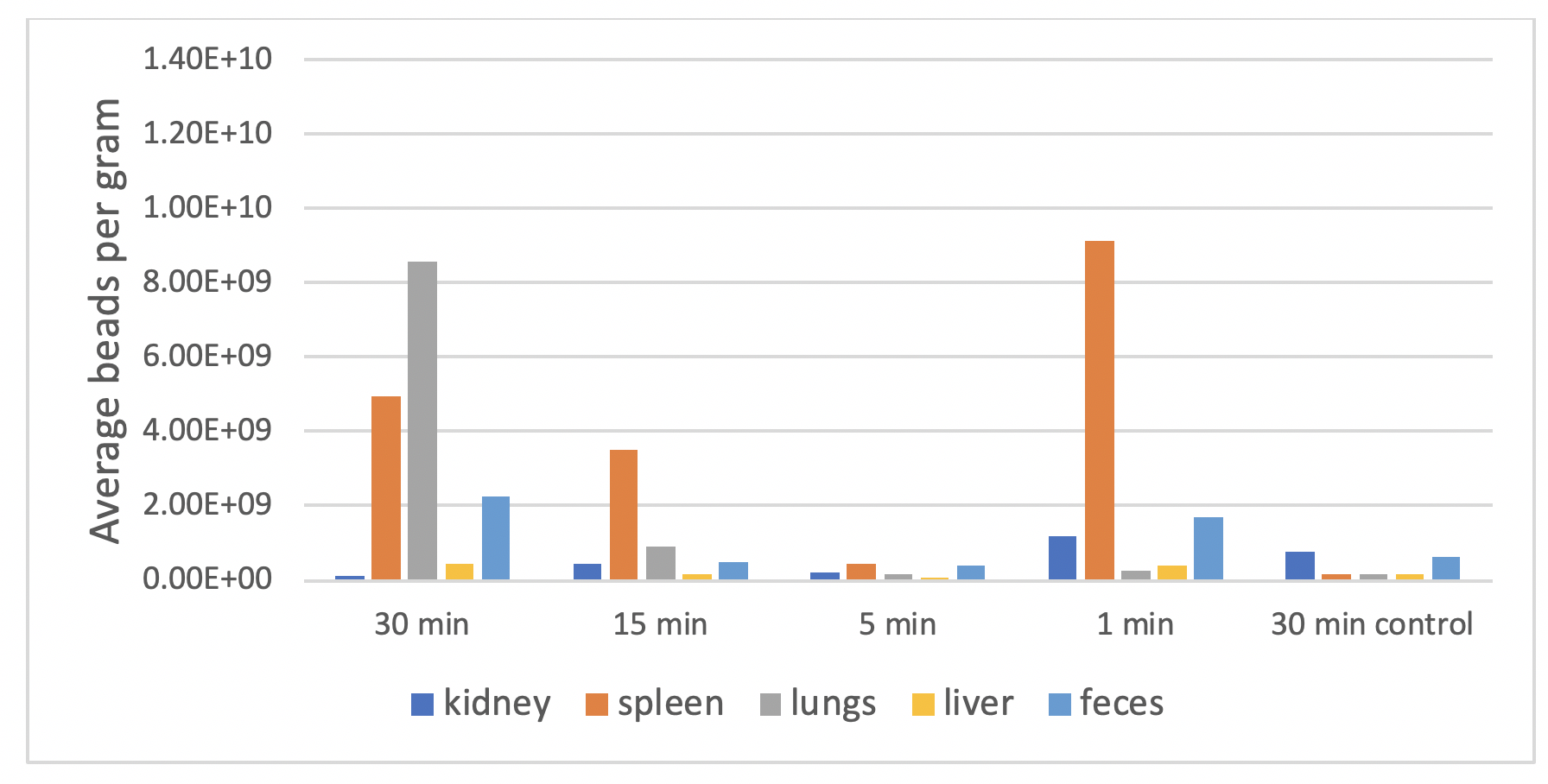
While current research involving nanocarrier transdermal delivery is mostly limited to lipids, metals and polymers, branched amphipathic peptide capsules (BAPC) have also been demonstrated to be a promising alternative. Personal communication with Dr. John Tomich’s lab at Kansas State University describe a mouse study with magnetic beads encapsulated in BAPC (BAPC-MB).
To test the transdermal delivery capabilities of BAPC, the tails of live mice were dipped in a solution of BAPC-MB for 1, 5, 15 and 30 minutes. After a 24-hour incubation period, the tissues were harvested for evaluation of tissue distribution.
This proof-of-concept experiment demonstrates that 50 nm BAPC-MB are rapidly absorbed transdermally and distributed to several different tissues. Although limited in scope, this work found accumulation of the beads in the lungs and spleen, with apparent excretion in the feces. Additional studies are underway to evaluate distribution to additional tissues, as well as inclusion of both oral and intravenous routes of administration.
24-Hour Incubation Period